Last month, I had the opportunity to attend Rare Disease Week on Capitol Hill with the EveryLife Foundation for Rare Diseases. This event was a unique opportunity to unite with rare disease patients, caregivers, and advocates from around the United States to discuss and advocate for federal legislative initiatives that may impact those living with rare disease. Advocates were given the opportunity to meet with the offices of members of congress from their home state to share their unique rare disease stories and perspectives, as well as to ask those congressional members to support rare disease patients through their legislative activities.
A disease or condition that impacts less than 200,000 people in the US is considered a rare disease. Dravet syndrome has been estimated to impact 1 in 15,700 individuals, which means there are likely between 16,000-20,000 individuals living with Dravet syndrome in the US. While the population living with Dravet syndrome is relatively small, there are currently over 7,000 rare diseases recognized by the National Institutes of Health that are estimated to impact between 25 to 30 million Americans. When rare disease voices unite on common initiatives, their collective message can be more powerful.
Legislative advocacy seemed overwhelming prior to attending Rare Disease Week on Capitol Hill, but the EveryLife Foundation works incredibly hard to make rare disease advocacy easy and approachable. They helped to develop legislative “asks” surrounding timely issues and activities impacting rare disease patients. EveryLife and their partner organizations developed educational materials and ‘one-pagers’ of information relevant to each legislative ask that could be readily shared with members of congress during advocacy meetings.
In addition to being much easier than I anticipated, the experience was also incredibly powerful. It was amazing to be gathered together with such a large group of individuals passionate about making change and speaking up for individuals with rare disease. There was broad representation across the spectrum of rare diseases, each with unique symptoms and challenges. However, despite the differences, there were so many moments of connection over shared experiences, and bringing all those voices together in unison really exemplified how, together, rare voices can be powerful change-makers.
I want to share some tools and tips I learned from the EveryLife Foundation that can empower the rest of the Dravet community to engage in advocating for important issues that impact this patient population.
- For each advocacy issue I describe below, you can use this ‘Take Action’ tool from the EveryLife Foundation to easily send a message to the US Senators and Representatives from your home state asking them to take action (see image below). The tool has wording to help describe the specific “ask,” and you can add in some specific information about your loved one with Dravet syndrome to help provide context on why this issue is important to you.
- If you would rather reach out to your federal senators and representatives personally via email or phone, find your federal and state officials and their contact information using the tool on this page.
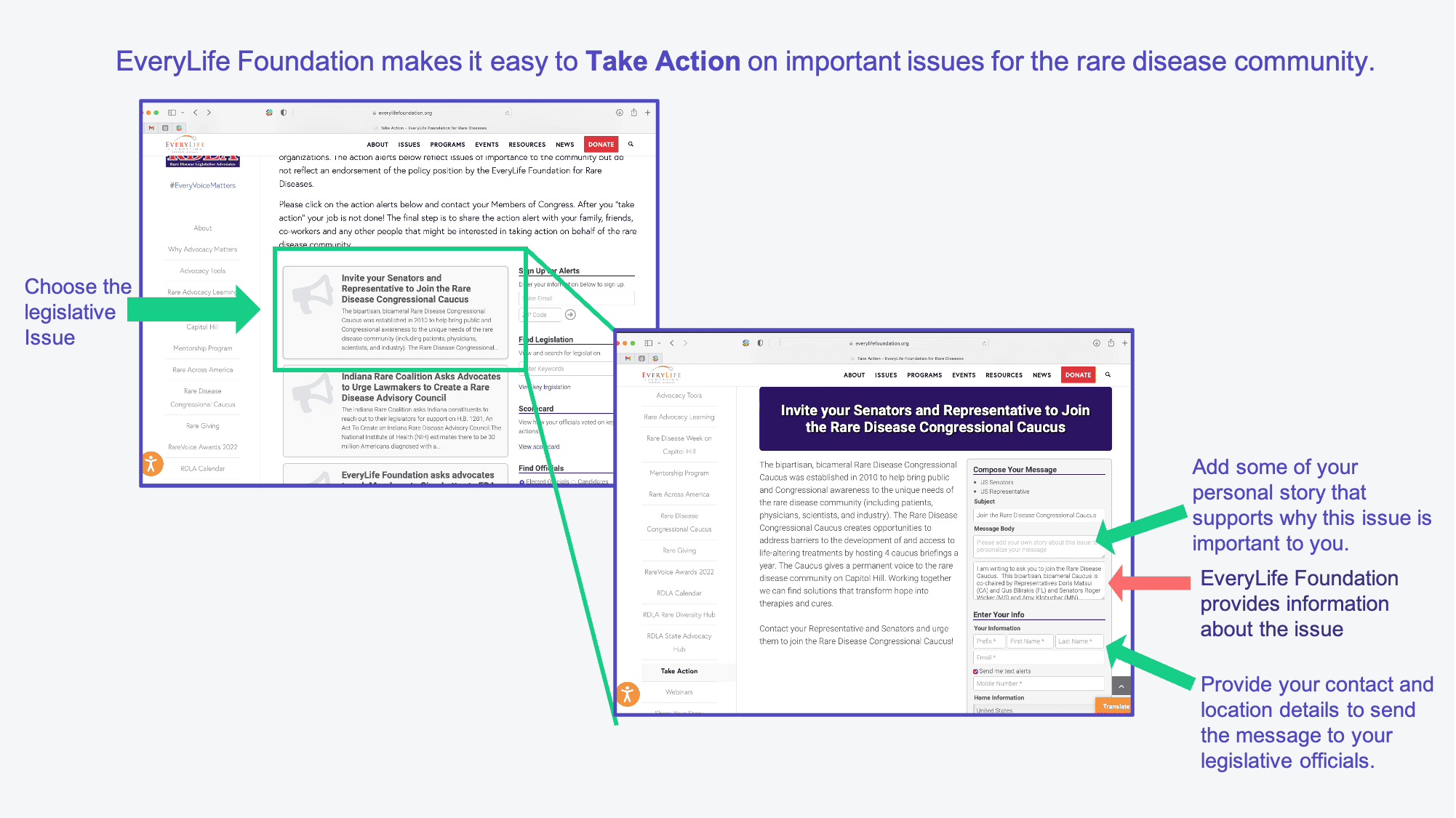
Take Action! Advocate for these issues that could impact patients with Dravet syndrome.
- Invite your legislative members to join the Rare Disease Congressional Caucus.
- This caucus is a group of Senators and Representatives that help to bring awareness to the unique needs of the rare disease community and ensure that the rare disease voice is permanently represented on Capitol Hill.
- You can find a listing of all the current members of the Congressional Rare Disease Caucus on the second page of this information sheet. If your respective members are not listed, ask them to join (and include the info sheet if you are not already using the EveryLife Take Action form). If they are already members of the Rare Disease Caucus, you can thank them for serving.
- Ask your members to sign this letter to the FDA in Support of a Rare Disease Task Force.
- The Congressional Rare Disease Caucus developed a sign-on letter asking the FDA to form an internal task force to review and inform agency-wide rare disease activities.
- While Dravet syndrome is one of the 5% of rare diseases that has FDA-approved treatments, there is still a great unmet medical need for patients. In the coming years it is likely that many potential therapeutics for Dravet syndrome will come through the FDA with applications for clinical trials and subsequent approvals for market. The FDA continues to make progress building strong organizational support for rare disease drug development, and this task force would further strengthen that infrastructure. Ensuring the FDA is approaching rare disease applications and approvals in a strong and consistent manner across the agency is critical for reaching improved treatment options for patients with Dravet syndrome.
- Ask your members to support the BENEFIT Act.
- The Better Empowerment Now to Enhance Framework and Improve Treatments (BENEFIT) Act helps to ensure that Patient-Focused Drug Development and patient experience data are incorporated into the risk-benefit framework when the FDA considers new therapeutic products.
- As noted above, there are likely to be a number of applications related to potential therapies for Dravet syndrome that come to the FDA in the near future. The FDA acknowledges that the approach to rare disease drug approval has to be unique in many ways and the FDA has made strides toward inclusion of the patient voice in their decision making. This act would help to close the remaining gap in the process, requiring documentation of how the patient voice is taken into account. DSF held a Dravet Syndome Externally-Led Patient Focused Drug Development (EL-PFDD) Meeting in February 2022 and developed the Voice of the Patient Report from that meeting. This act would further ensure that the FDA is incorporating that information, developed directly from the voice of families living with Dravet syndrome, in their decision making process.
- Another patient advocacy group, Parent Project Muscular Dystrophy, developed this BENEFIT Act information sheet. You can follow the Take Action tool from EveryLife foundation to a unique form page linked through Parent Project Muscular Dystrophy, or you can email your senators and representatives separately while attaching the BENEFIT Act information sheet for them to learn more details.
I hope you will join DSF in raising our collective voices for these legislative initiatives. It really is a simple process that can make a big difference when we all do it together. Stay tuned to DSF through our social media, newsletter, and blog posts to hear about advocacy opportunities and more ways to get involved in legislative advocacy in the future.
If you have questions, need help navigating any of this information, or coming up with facts to include about Dravet syndrome in your legislative outreach, you can contact me: Veronica Hood, PhD; veronica@dravetfoundation.org






