Importance of Research
Learn about the value of research to the Dravet syndrome community and how to read a research paper
The Importance of Research for Dravet Syndrome
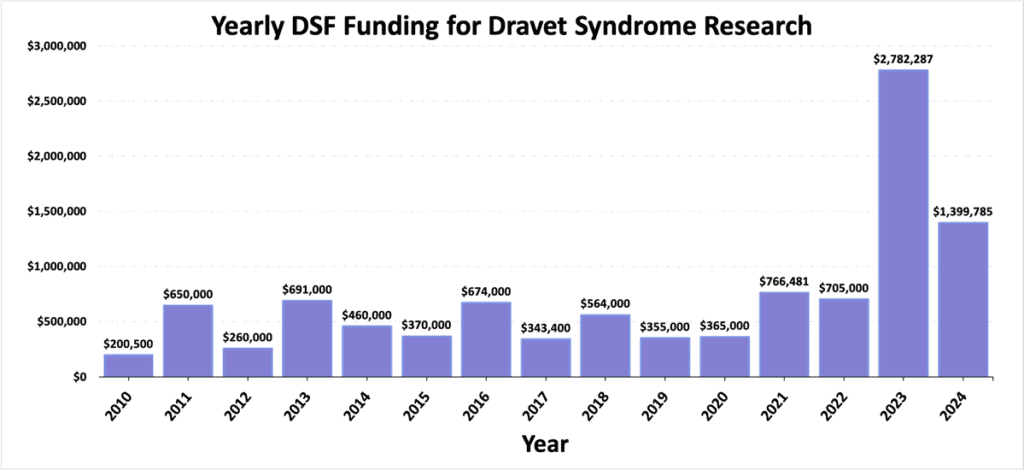
Severe myoclonic epilepsy of infancy (or ‘SMEI’) was first described in 1978 by Dr. Charlotte Dravet and was renamed in 1989 as Dravet syndrome. By 2001, researchers had discovered that a primary cause of Dravet syndrome was related to mutations in SCN1A, a gene responsible for making sodium channels that affect electrical activity in the brain. Despite this major advancement in the scientific understanding of the cause of Dravet syndrome, research was still slow to advance. By 2009, families had come together to form the Dravet Syndrome Foundation (DSF) in an effort to spur and support research efforts towards a better understanding of the underlying causes, the presentation and progression, and the best treatment approaches for Dravet syndrome. DSF has made research a core priority, funding over $11.3 million of research efforts since 2009. Through the combined efforts of DSF, scientists, clinicians, industry, government, and patient families, research has led to advancements in the care and treatment of those living with Dravet syndrome. While much has been accomplished, there is still great unmet need for patients and their families, underlining the importance of continuing to fund, advocate for, and participate in further research efforts.
The Importance of Basic Science Research
The initial link between DS and mutations in a gene, SCN1A, came from studies of genetic samples from human patients. Following that discovery, scientists used an array of basic science tools and studies to unravel WHY mutations in SCN1A might result in the symptoms of Dravet syndrome. Breakthroughs using cells, fish, and mice have revealed the role of this gene in specific cells in the brain and increased our understanding of how SCN1A is regulated. The development of animal models that mimic the genetics of Dravet syndrome have allowed researchers to investigate more about the cause and symptoms of Dravet syndrome and to screen drugs and therapies that could translate to human treatments. Research advancements have uncovered biological details that are guiding the development of gene-targeted therapies that attempt to correct the root cause of Dravet syndrome.
The Importance of Clinical Research
Clinical research, or research directly involving humans, works hand-in-hand with basic research to improve outcomes for patients. Clinical research seeks to better understand a condition or disease or to investigate methods to intervene to improve treatment for a condition or disease. As our understanding of the cause and progression of Dravet syndrome in human patients has grown over the years through clinical research, it has improved standards of clinical care for patients as well as informed the direction of basic science research to focus on particular symptoms and interventions. In turn, basic science discoveries have led to interventional clinical trials of new medications and therapies for Dravet syndrome.
Research has Changed the Landscape for Dravet Syndrome
Basic and clinical research continue to improve outcomes for individuals with Dravet syndrome. When DSF was founded, there were no specific treatments for Dravet syndrome and research studies were still limited. The breadth and depth of research knowledge has significantly expanded since that time, further stimulating additional research efforts and leading to improved clinical care for patients.
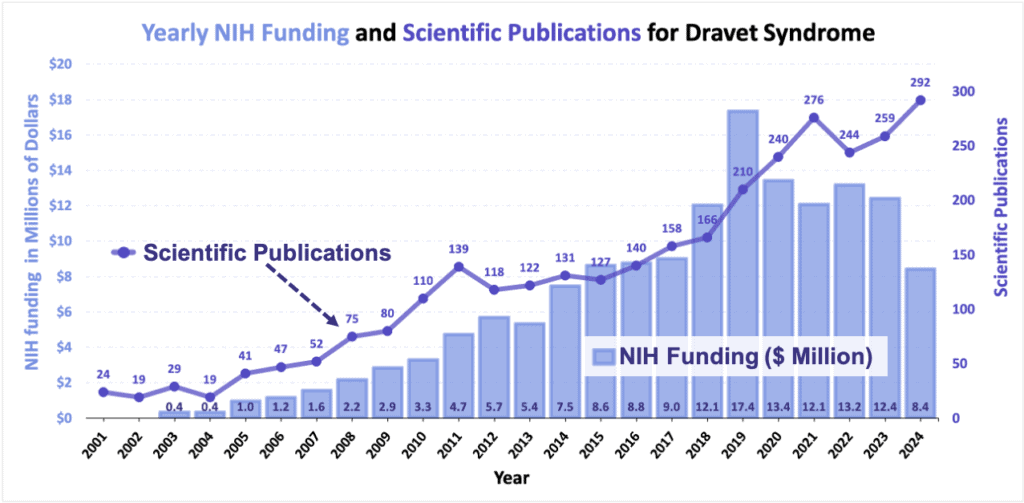
The National Institutes of Health (NIH), the government agency responsible for supporting biomedical and public health research, has also increased funding for grants related to Dravet syndrome.
- DSF grants provide ‘pilot funding’ that allows researchers to build enough preliminary data to apply for larger funding. The NIH plays a crucial role in advancing research projects further than DSF funds may be able to do.
- NIH funding for projects related to Dravet syndrome were similarly lacking in the early 2000s, with the first relevant funding occurring around 2003.
- In 2008, the NIH directed about $2 million towards Dravet-related research.
- Funding for Dravet-related projects continued to grow, frequently exceeding $10 million dollars each year.
The number of yearly scientific publications with relevance for Dravet syndrome have increased greatly over time.
- Prior to 2001 when the initial gene associations were made, there were less than 5 publications each year with a focus on Dravet syndrome or SCN1A.
- In 2024, there were over 290 papers published related to Dravet syndrome or SCN1A.
These increases in scientific funding and publications are representative of major advancements to the field that have subsequently impacted patient care. A few key examples of such advancements are outlined below:
- The most recent studies attribute over 95% of Dravet syndrome diagnoses to mutations in the SCN1A gene; increased recognition of the genetic component of Dravet syndrome has led to swifter access to genetic testing and earlier diagnoses.
- Importantly, earlier diagnoses allow for avoidance of contraindicated medications (sodium channel blockers) which have been shown through research studies to worsen seizures and long-term outcomes for patients with Dravet syndrome.
- Research also helped us to understand that the mutations that cause Dravet syndrome decrease the function of sodium channels in the brain, explaining the need to avoid medications that further block sodium channel function.
- Clinical trials for fenfluramine led to FDA approval in 2020 and fenfluramine has led to better seizure control than previously measured in patients with Dravet syndrome (on average).
- Zebrafish drug screens, supported in part by DSF funding, uncovered several serotonin-modulating drugs that may be effective for seizure control- a standout from those studies, clemizole, is currently in clinical trials.
- Several other serotonin-modulating drugs are in development or clinical trials for Dravet syndrome.
- In 2020, the first patient with Dravet syndrome was dosed with an anti-sense oligonucleotide, called zorevunersen or STK-001, in a clinical trial in hopes to increase healthy levels of expression of SCN1A in the brain. Clinical trials for zorevunersen are expected to begin Phase 3 in 2025.
- Several other gene-based approaches are being developed by academic and industry researchers.
Real-time studies of how the signs and symptoms of Dravet syndrome present in patients as they age (also called “Natural History Studies”) are currently underway. These types of studies can lead to improved clinical care as well as supplement and inform clinical trials for new treatments.
The Importance of Your Involvement in Research
Scientific advancements take a lot of time, money, and effort, but things move faster when everyone works together. The patient and caregiver voice are invaluable to direct research efforts where they will matter most to those living with Dravet syndrome. Meaningful results and the success of clinical studies are dependent upon patient and caregiver participation; a population that is “clinical trial ready” is essential to spur additional drug development efforts. Moreover, the determination of families to fund research has made a major impact, which is reflected in the over $10.6 million that DSF has directed towards research since 2009. In addition to the direct funding of research, DSF supports research in a variety of other ways, including acting as a hub to convene all the stakeholders with interest in moving research for Dravet syndrome forward. Staying connected to DSF will help keep you up-to-date on research efforts and opportunities for participation.
Get Involved in Research
- DSF regularly updates this page with actively enrolling clinical study information, as well as an overview of the treatment pipeline for therapies that are near or in human trials for Dravet syndrome.
- DSF also helps to raise awareness of active study opportunities through our Family Network, newsletter, caregiver support groups, Decoding Dravet blog, webinars, and social media channels.
- If you find a study you are interested in, discuss it with your loved one’s health care providers.
- Filling out a survey or questionnaire is a low risk way to lend your voice to research.
- Opportunities for remote participation in studies that support or guide research efforts are also a great chance to get involved.
- DSF raises awareness of surveys and similar studies through our Family Network, newsletter, caregiver support groups, Decoding Dravet blog, webinars, and social media channels.
- DSF’s website is a rich source of information about Dravet syndrome, backed by scientific research, where you can learn about the genetics and biology of Dravet syndrome, comorbid symptoms, expert treatment recommendations, and gene therapies in development.
- Join DSF webinars and attend the yearly Day of Dravet events and biennial Patient and Professional Conferences to hear about active research efforts and to ask questions directly to the researchers doing the work.
- DSF offers an extensive library of recordings on an array of research topics from past webinars and conferences.
- Ready to delve deeper into reading research studies? Check out the Decoding Dravet blog series featured at the bottom of this page to learn tips on reading directly from scientific publications.
- DSF has directed over $10.6 million dollars to research because of the amazing fundraising efforts of the patient community. Incredible progress has occurred, but there are still so many unmet needs for patients with Dravet syndrome and their families.
- There are many ways to fundraise with DSF to help reach better treatments sooner. Get started with a personal campaign page or learn about planning a larger event!
How to Read a Scientific Paper: 3-Part Blog Series
How to Read a Scientific Paper; Part 1: Anatomy of a Research Article
Read More →How to Read a Scientific Paper; Part 2: Breaking Down the Information
Read More →Clinical Trials & Pipeline
Learn about current trials and how to participate
Gene Therapy Info
Learn about potential gene therapy options for Dravet
SUDEP Registries
Learn about SUDEP and registries





